Density is the amount of mass in a volume. The more dense a substance is, the heavier it is for the same amount of volume.
The formula for density is:
`text(density) = frac(text(mass))(text(volume))`
Density is measured in g/cm3 or kg/m3.
A cuboid of lead, 3cm by 5cm by 2cm, has density of 11.34 g/cm3. What mass is the cuboid?
Volume = 3 x 5 x 2 = 30cm3.
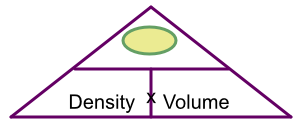
Cover up mass: left with density x volume
30 x 11.34 = 340.2g
Answer: 340.2g
The density of water is 1000 kg/m3. A swimming pool has been constructed on the top of a building. The swimming pool is 1.5m deep throughout, and is 10m by 15m. What mass of water will be contained within the pool?
The volume of the pool is 1.5 x 10 x 15 = 225m3.
From the triangle, cover up mass: left with density x volume:
225 x 1000 = 225000Kg, os 225 tonnes.
Answer: 225 tonnes